Describe the Basic Structure of an Atom.
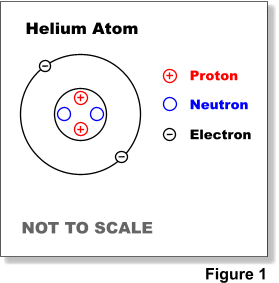
Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. There are five basic atomic models which have contributed the structure of the atom itself.

Structure Of An Atom Class 9 Science Notes Leverage Edu
Diatomaceous earth is a very fine powder which can be spread on the soil or sprayed on the plant in a solution of insecticidal soap and water.

. Describe and model the structure of the atom in terms of the nucleus protons neutrons and electrons. An atom consists of a nucleus of protons and neutrons surrounded by a group of orbiting electrons. The nucleus center of the atom contains the protons positively charged and the neutrons no charge.
The protons and neutrons make up the nucleus of the atom which is surrounded by the electrons belonging to the atom. Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells. 2 Define free electron.
The nucleus center of the atom contains the protons positively charged and the neutrons no charge. The numbers of subatomic particles in an atom can. The positively charged components of the atom.
Its nucleus contains protons and neutrons. The outermost regions of the atom are called electron shells and contain the electrons negatively charged. The energy levels are often called rings see more discussion of the Bohr model below.
Atoms are the basic unit. Atoms are made up of small particles called protons neutrons and electrons. The density of ethanol is 0789 gmL.
A atom is not circular. The mass of ethanol needed to fill the tube is found to be 4523 g. And the reason you see them drawn with a circle around the nucleus is because theyre showing you the probable path the electrons are taking around the nucleus.
The part of the atom that houses the protons and the neutrons. Atoms have a nucleus and electrons. Each shell can occupy a certain number of.
3 pts b Explain the difference between an atom and an ion. Structure of diatomaceous earth damages and dehydrates the bodies of many insects and many soil pests. Extension Activity 1 - Construct a 3D Model of an Atom.
Electrons are attracted to the protons in the nucleus but are moving so quickly they fall toward it orbit rather than stick to protons. In this article we familiarize you with the basic structure of an atom. Your protons and neutrons are located in the center of the atom with the electron orbiting outside.
Atoms consist of three basic particles. Outside of the nucleus are energy levels also called shells which contain one or more electrons. Daltons Billiard Ball Solid Sphere Model JJ.
Describe the structure of an atom with respect to the arrangement of protons neutrons. The atoms basic structure is composed of three particles which are the protons that are positively charged neutrons that have no charge and electrons that are negatively charged. The outermost regions of the atom are called electron shells and contain the electrons negatively charged.
Here the electrons are negatively charged while the protons are positively charged. Basic Diagram of an Atom. The electrons are negative charged.
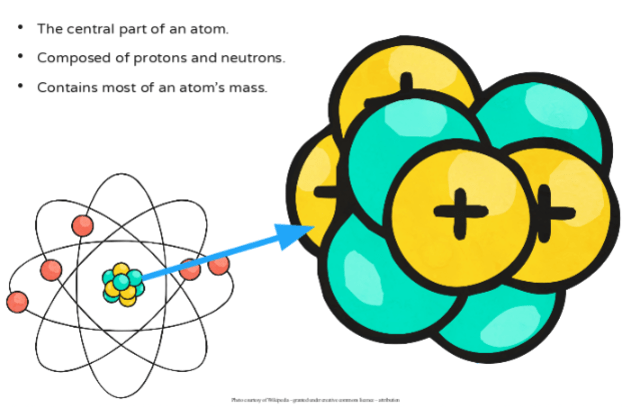
The smallest component and the most basic part of matter that still retains its identity. These particles have different positions inside the atom. In the center of an atom is the nucleus made of protons and neutrons.
The structure of atoms is a small electron - orbiting the atoms nucleus which contains the much bigger proton and neutron. The law stating that reactants will always combine in set whole number ratios. It remains active in the soil for many years.
6 pts total a Fully describe the basic structure and components of an atom. The Electronic Structure of an Atom. Protons electrons and neutrons.
The protons and neutrons are at the center of the atom. The three parts of the atom are protons positively charged neutrons neutral charge and electrons negatively charged. The basic structure of an atom is made up of neutrons protons and electrons and its atomic number is calculated by adding up the number of protons and neutrons in the atoms nucleus.
Protons electrons and neutrons. Primarily the atomic structure of matter is made up of protons electrons and neutrons. Law of multiple proportions.
Answer the following questions on atoms and ions. A 250-cm-long cylindrical glass tube sealed at one end is filled with ethanol. Calculate the inner diameter of the tube in centimeters.
The smallest possible amount of matter that still retains its identity as a chemical element now known to consist of a nucleus surrounded by electrons. The basic structure of an atom includes a tiny relatively massive nucleus containing at least one proton and usually one or more neutrons. Homework 2 describe the basic structure of an atom including the characteristics mass charge of protons neutrons and electrons.
The center of an atom is the nucleus and one or more electrons surrounding the nucleus. The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Protons and neutrons form the atomic nucleus.
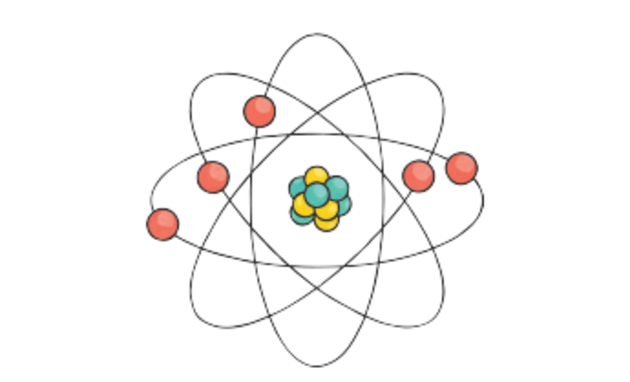
The shells of an atom are numbered 12 3 and so on starting from the one closest to the nucleus. Electrons are arranged around the nucleus in the shells of an atom. UNIT 31 REVIEW QUESTIONS 1 Describe the basic structure of the atom.
Atoms consist of three basic particles. Learn about the basic structure and the atomic number of an atom in this article. Each atom in its normal state has an equal number of electrons and protons and since their charges are equal and opposite they cancel leaving the atom electrically neutral ie with zero.

Atomic Structure And Electrons Structure Of An Atom What Are Atoms Neutrons Protons Electrons Youtube

Introduction To Structure Of Atom Proton Neutron Electron With Examples
What Is Atomic Structure Definition Meaning And Resources
What Is Atomic Structure Definition Meaning And Resources

Draw The Structure Of Atom And Explain About It Study Com
The Structure Of The Atom Boundless Chemistry

The Structure Of An Atom Protons Neutrons Electrons Chemtalk

Particles In The Atom Atomic Structure 1 1 1 Cie A Level Chemistry Revision Notes 2022 Save My Exams

Chemical Bonding Atomic Structure And Bonding Britannica

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

2 1 2 2 Atomic Structure Sl Youtube

Q2 Draw The Basic Structure Of Lido

The Structure Of An Atom Protons Neutrons Electrons Chemtalk

Components Of An Atomwhat Makes Up An Atom Orbiting Around The Nucleus Are The Electrons Electrons Atom Atomic Structure Birthday Captions Instagram

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets

Draw The Structure Of Atom And Explain About It Study Com

Atomic Structure Electrons Protons Neutrons And Atomic Models